Reaction cascade model
All the solid-state reactions are decomposed into pairwise reactions using the principles developed in our previous works [BianchiniNM2020] and [MiuraAM2020]. The synthesis reactions are assumed to be performed under a gas reservoir (\(O2\), \(CO2\), \(N2\), \(NH3\)) by which gas partial pressures are kept constant. Therefore, the relevant grand potential change are calculated as the reaction driving force \(\Delta \Phi_{rxn} = \Phi_{products} - \Phi_{reactants}\). The chemical potential of gas species is calculated using the relation \(\mu (T) = \Delta G_f (T) + RT\ln p\), where \(\Delta G_f(T)\) is the experimentally determined Gibbs free energy of formation, \(R\) is the gas constant, and \(p\) is the effective partial pressure of the gas in normal atmospheric conditions.
The following figure demonstrates an example of the pairwise reaction cascade construction. Starting with precursor compounds A, B, and C, we first enumerate all possible pairs of precursor compounds, e.g., \(\{\ce{A}, \ce{B}\}\), \(\{\ce{A}, \ce{C}\}\), and \(\{\ce{B}, \ce{C}\}\). For each pair of precursors, we identify all possible pairwise reactions (e.g., \(\ce{A} + \ce{C} \to \ce{AC}\)) by enumerating all MP entries. These pairwise reactions are normalized per mole of non-gas elements (e.g., the target compound \(SrTiO_3\) is normalized by 2). We select the reaction with lowest reaction driving force \(\Delta \Phi_{rxn}\) and use it to consume as much precursor compounds as possible. Note that the reaction driving force is not necessarily negative to tolerate some uncertainties in our calculated thermodynamics. This process is repeated until no possible pairwise reaction could be constructed.
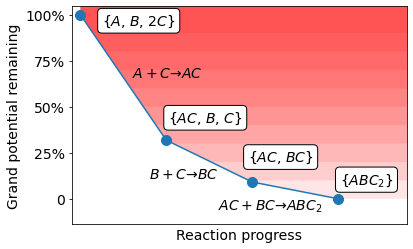
Schematics of the cascade model. We start with the precursors, and each time choose the reaction with lowest energies (normalized by metal cations, or grand potential). The process is repeated until there is no more possible pairwise reactions.
- BianchiniNM2020
Bianchini, Matteo, et al. “The interplay between thermodynamics and kinetics in the solid-state synthesis of layered oxides.” Nature materials 19.10 (2020): 1088-1095.
- MiuraAM2020
Miura, Akira, et al. “Observing and Modeling the Sequential Pairwise Reactions that Drive Solid‐State Ceramic Synthesis.” Advanced Materials (2021): 2100312.